What is the Gateway?
The ESG is a portal that facilitates communication between the FDA and the pharmaceutical industry by providing a secure digital environment to submit regulatory documents for review, which operates on a similar principle to Fully-Verified’s KYC provider service.
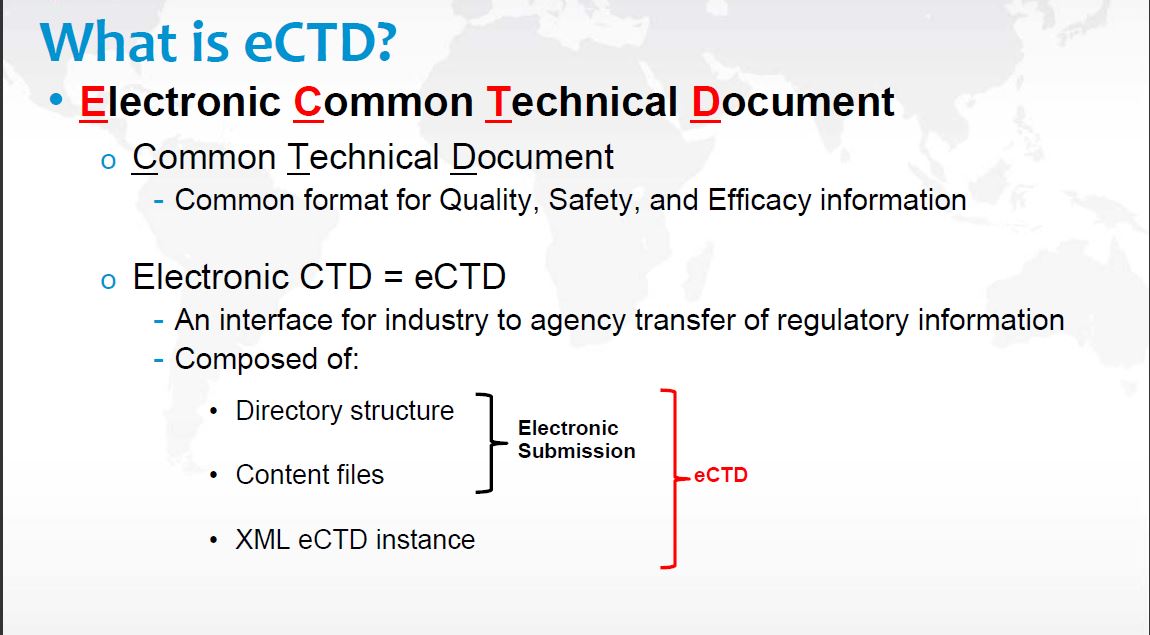
The FDA Electronic Submissions Gateway is an Agency-wide solution for accepting electronic regulatory submissions and is the required method of transmission for eCTDs 10GB or less.
We are certified ESG Production provider.
Plan ahead for first-time submissions via the Gateway
Gaining access to the ESG is a multistep process that’s too important to rush. Based on our experience, we’d recommend setting up your account at least 2 months before the due date for your first electronic submission.
We specialize in eCTD submission, the new FDA registration paradigm. We submit or update DMF and BPF.
Our ESG eCTD FDA Production was certified and validated by FDA. If you have any question or concerns, please contact us at jsc@fdaconsultantnusagent.com